TITTLE : TEST ON
THE EFFECT OF DIFFERENT CONTENTS ON THE
CHARACTERISTICS OF SUPPOSITORY
FORMULATION.
Introduction
Suppository is a solid formulation with various sizes and
shape suitable for rectal administration. A good suppository should melt after
administration into the rectum and release drugs for local or systemic effect.
The drugs should be distributed in a suitable suppository
base. A good base should be non-toxic, no irritation, no reaction with the drug
and easy to be form into a suppository. A different base composition will
influence the rate and limit of drug release from the suppository.
In this experiment, the effect of different base composition
to the physical characteristic of formed suppository and the effect of drug
release will be studied.
Apparatus
Weighing scale
1 weighing boat
Spatula
1 beaker (50ml) and 1 beaker (100ml)
Hotplate
1 measuring cylinder (5ml)
1 set of suppository mould
Water bath (37°C)
1 dialysis bag (10cm)
2 threads
1 glass rod
1 pipette (5ml) and pipette bulb
1 plastic covet
Spectrophotometer UV/Vis
Materials
Polyethylene glycol (PEG) 1000
Polyethylene glycol (PEG) 6000
Paracetamol
Procedure
1.
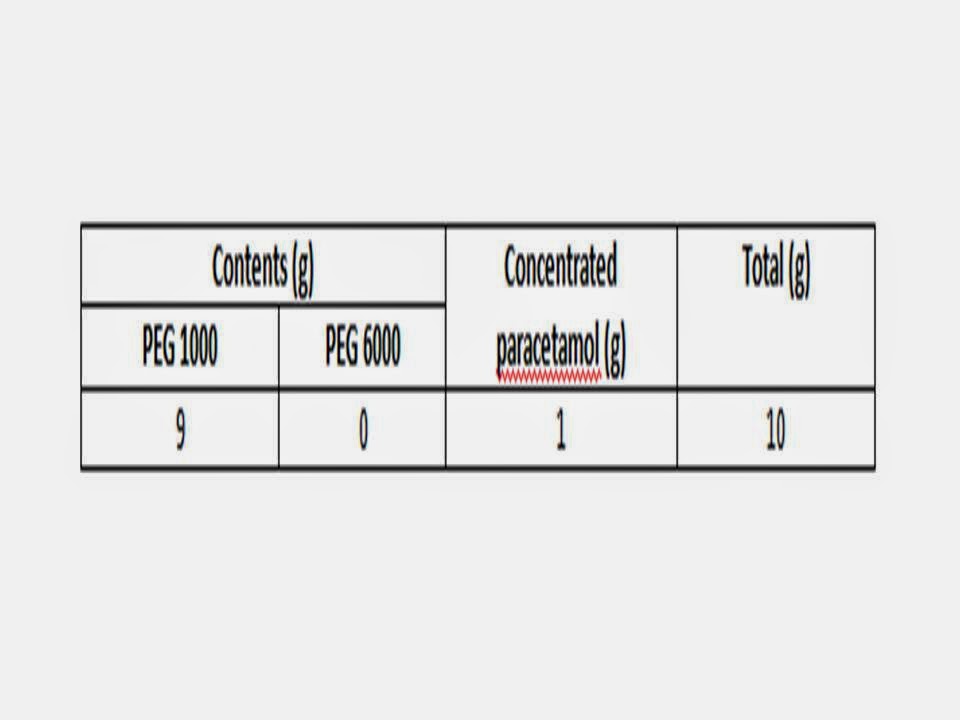
A concentrated solution of paracetamol was
prepared (10 g on 5 ml of distilled water).
2.
A paracetamol suppository (10g) was prepared
using the formula.
1.
The suppository was formed using the
suppository-mould. The shape, texture and the colour of the suppository were
compared.
2.
One suppository was inserted into a beaker
containing distilled water (10 ml, 37°C) and the time taken for the suppository
to melt was recorded.
3.
One suppository was placed into a dialysis bag
and both ends of the bag were tied. The bag was placed into a beaker (100ml)
containing distilled water (50ml) which has been heated to 37°C.
4.
At each interval of 5 minutes, an aliquot sample
(3-4ml) was pipette and the release of paracetamol from the suppository was
determined using the spectrometer UV-visible. The distilled water was stirred
before the sample is taken.
RESULTS AND DISCUSSIONS
Compare the physical appearance of the
suppositories and give explanation
The different quantities of
Polyethylene Glycol (PEG) which are PEG 1000 and PEG 6000 for each base
formulation of suppositories will produce different physical characteristics of
those suppositories. Based on the experiment, all the suppositories have the
shape of a bullet since the mould that is being used is of this shape.
For the formulation that has the
highest amount of PEG 1000, which means that it has the lowest quantity of PEG
6000 shows greasy surface compared to the other suppositories. In other words,
the higher the quantity of PEG 6000 in a formulation, the less greasy the
suppository will be. Besides, all the formulation of suppositories has smooth
texture. As for the hardness, all the suppositories produced are hard.
Theoretically, the higher amount of PEG 1000 compared to PEG 6000 will give
softer suppository and vice versa. The inaccurate result may due to some errors
occurred throughout the experiment. The softer suppository provides ease to be
administered to the patient compared to the hard one and it may lead to pain in
the process of administering. Besides, it will easily melting within the body
and also dissolves in body fluids.
For the colour of suppositories,
since the active ingredient that we used is paracetamol which is white in
colour, the colour of the suppositories produced also white but differ in the
transparency degree. The colour changes from evenly white to uneven white with
decreasing PEG 1000. This means that the formulation with the lower amount of
PEG 1000 is more transparent compared to the others.
2. Plot a graph of
time needed to melt suppository against the content of PEG 6000 in the
formulation. Compare and discuss the result.
Based on the graph, 9g PEG 6000 melts the longest followed
by 6g, 0g and 3g. Theoretically, less PEG 6000 will melts first. In this
experiment, 0g is not the fastest to melt instead, 3g is. Therefore, there may
be some mistake occurred and as we are group 1, we will discuss about our
experiment only. During experiment, the temperature of water bath is not
constantly at 37°C
because there are many other groups using the same water bath hence the
temperature of water inside beaker may be lower than 37°C. The deviation between our group and group
5 is considered big that is 8.1 and their group melts at 54 minutes. This may
be due to the cooling of suppository in freezer take too long hence the
suppository freezes and take time to defrost before melts.
3.
Plot
a graph of UV absorption against time. Give explanations.
From the graph obtained above, the UV absorption at 520nm is the highest
during 10 minute. The UV absorption at 520nm is in an increasing order except there
is slightly drop during the 25 minute and 60 minute. Theoritically the longer
the period the dialysis bag stays in the solution, the more the amount of
paracetamol diffuses out of it, and hence the higher the value of UV absorption
because more UV light is absorbed. In this experiment, dialysis bag resembles the lipid bilayer membrane while
the distilled water simulates the human plasma which represents the drug
diffussion mechanism in our body. At 37 oC, the drug in the
suppositories will diffuse into the systemic circulation due to the concentration gradient
between the melted suppositories in the dialysis bag and dilutes solution
outside the dialysis bag.
This may due to some error in handling the sample and inapproriately use of
machine. Other reason may be impurities in the sample container due to
incomplete washing.
4. Plot a graph of UV light absorption against
time for the suppository formulation which contained different compositions.
Give explanation.
Mean:
I=0.098
II=0.075
III=0.027
IV=0.020
From the graph above, the suppository I has the highest UV absorption at
520nm, which means it has the highest concentration of paracetamol in the
distilled water, followed by formulation II, III and IV. The results are tally to the theoretical
value because different formulations of suppository give rise to different rate
of drug release profile. The releasing rate of paracetomol is measured by
spectrometry. Theoretically, the greater amount of PEG 6000, the slower the
drug will be released from suppository. This is because the higher amount of
hydrogen bonds formed between the base molecules and the paracetamol molecules
hence the drug molecules (paracetamol), to be held stronger in the formulation
and thus exert a lower releasing rate. Although the graph obtained from this
experiment is very fluctuated, however generally there showed an increased
concentration of paracetamol over time and the rate of drug releasing decreased
with increased amount of PEG 6000 in each formulation. The graph obtained
should be increasing initially and then become constant gradually as all the
drug is released into the water..
The fluctuation of the
graph may be due to several errors occurred while handing the UV spectrometry
or human errors, such as the incorrect way in the suppositories preparation or
the presence of impurities in the formed suppositories. Besides, the uneven
stirring process before the content of the mixture is pipetted also can
contribute to the inaccuracy of the results which forming a few small
fluctuations in the line graph. Some precaution steps should be carried out to
obtain an accurate result. The surface of dialysis bag should be cleaned before
immersing it into the distilled water and suppository should be filled into the
bag carefully to prevent breakage of the bag.
5. What is the function of each
ingredient that is used in the preparation of these suppositories? How does the
usage of different content of PEG 1000 and PEG 6000 affect the physical properties
of suppository formulation and rate of releasing of drug from it?
Paracetamol is active ingredient in
the suppositories. Paracetamol is used as analgesic and antipyretic. It appears
as white, odourless and light powder. PEG 1000 and PEG 6000 are the bases for
the active ingredient, paracetamol of the suppository. They allow a smoother
drug delivery of the suppository into the rectal. They also allow the
absorption of paracetamol by the membrane to occur. A suitable combination of
PEG allows an optimum drug releasing to occur, in which the drug will not be
held strongly in the base and can be easily released. This is important to
allow an optimum drug bioavailability to take place as the drug can be absorbed
by mucosa membrane of the rectal.
The physical characteristic and the
rate release of the suppository preparation can be interfered by the different
combination of PEG1000 and PEG6000. As the proportion of PEG6000 increases, the
drug becomes more difficult to be released from the suppository. Besides, the
suppository also will become hard, crystal like and with a clear white colour.
Proper combination of base should be determined to achieve a balance between
hydrophilic and lipophilic characteristics.
CONCLUSION
From the experiment, we can
conclude that the higher the amount of PEG 6000 in the formulation of
suppository, the lower the rate of drug released.







No comments:
Post a Comment